Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Satoru; Kawamura, Katsuyuki*
JNC TN8400 2001-005, 41 Pages, 2001/04
A correlation between molecular structure and a vibrational spectrum of interlayer water in Na-smectite was investigated by means of Molecular Dymamics (MDs) simulations. Detailed comparison of simulation results with IR spectroscopic observations for the water-smectite system indicated good agreement. Internal vibrational spectra of water were obtained by the Fourier transformation of velocty auto-correlation function of hydrogen atom. A stretching vibrational spectrum of interlayer water consisted of a broad band with a peak top around 3400cm and a sharp peak around 3650 to 3700cm
and a sharp peak around 3650 to 3700cm . The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O
. The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O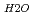 -O
-O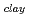
 3.0
3.0  -O
-O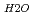 = ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
= ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
Ashida, Takashi; ; Sato, Haruo; ; Kitamura, Akira; Kawamura, Kazuhiro
JNC TN8400 99-083, 63 Pages, 1999/11
Studies on the chemical and migration behaviour of radionuclides were carried out in the Quantitative Assessment Radionuclide Migration Experimental Facility (QUALITY)for assuring the relaiability and for improving the propriety of data concerning nuclide migration used in the Second Progress Report for the geoloical disposal of high-level radioactive waste. Five studies for solubility, sorption and diffusion concerning nuclide migration were carried out. The overview of each study and the result is as follows: (1)Study on Effect of Carbonate on Np Solubility. Solubilities of Np(IV) were measured as functions of pH and carbonate concentration under reducing conditions. The obtained data could be well described by considering two hydroxo-carbonate complexes, and those stability constants were estimated and compared with the literature data. Consequently, the data obtained in this study were similar to the literature data. (2)Study on Effect of Carbonate on Np Sorption on Bentonite. Distribution coefficients (Kd) of Np(IV) on smectite were measured as a function of carbonate concentration. The obtained Kd values were approximately constant over the carbonate concentration (total carbon concentration 0.04-0.15M). The results of desorption tests by 1M KCl and HCl at the end of sorption experiments showed two different desorption behaviour; Np(IV) was well removed by HCl for the experiments in low carbonate concentration and by KCl for those in high carbonate concentration. (3)Distribution Coefficient Measurements for Cs, Pb and Cm on Rocks. Distribution Coefficients for Cs, Pb and Cm on Japanese major rocks (basalt, mudstone, sandstone, granodiorite and tuff) were measured as a function of ionic strength. The obtained Kd values were either the same orders or higher compared with data used to both fresh and saline groundwater systems in the Second Progress Report. This indicates that the Kd data used in the Second Progress Report are either proper or conservative. ...
Oda, Chie; Ikeda, Takao*; Shibata, Masahiro
JNC TN8400 99-073, 112 Pages, 1999/11
In the safety assessment for geological disposal of high-level radioactive wastes (HLW), distribution coefficients (Kd) and diffusion coefficients of radionuclides are used to estimate the migration of radionuclides in a near-field of repository.  Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
 10
10 [ml/g], 10
[ml/g], 10
 10
10 [ml/g] and 10
[ml/g] and 10
 10
10 [ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10
[ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10 [m
[m /sec] and l0
/sec] and l0 [m
[m /sec] for dry densities of 0.4[g/cm
/sec] for dry densities of 0.4[g/cm ] and 1.0[g/cm
] and 1.0[g/cm ], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO
], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.